A 26.7 Gram Sample Of Which Element Grms To Grms Clcultor Stoichiometry
In order to calculate the number of atoms in a one gram sample of any substance, you must calculate the number of moles of that substance based on its molar mass. 11 which sample at stp has the same chemical properties as 10. Calculate the volume of a 26.7 g sample of benzene.
Grams To Grams Calculator Stoichiometry
The element with a sample of 26.7 grams that has a volume of 3.00 cubic centimeters at room temperature can be found by using the concept of density. Grams of si(s) (3) 5 grams of al(s). Ar has a molar mass of about 39.95 g/mol.
(1) cr (2) cd (3) nb (4) ni.
We can use the following. The density (ρ) of anything is its mass (m). Find the number of moles for each element by dividing the mass present with the relative mass of the atom. Density for this sample is 26.7g/ 3cm3 = 8.9 g/cm3.
Density is equal to the mass over volume. The element is titanium, ti. Let us suppose that a given mass of a compound or element is 'x' grams, and the molar mass of the compound or element is 'm' g/mol. Grams of al(s) at stp?

Determine element using grams and atoms of certain substance YouTube
The number of moles of the compound or element can.
Table s on the reference table shows density values for all the elements. Densities of some substances at 20°c substance density (ml. Here’s the best way to solve it. 0.668 moles of atoms are in 26.7 g ar and za has no moles because it does not exist.

26.7 Grams To Ounces Converter 26.7 g To oz Converter
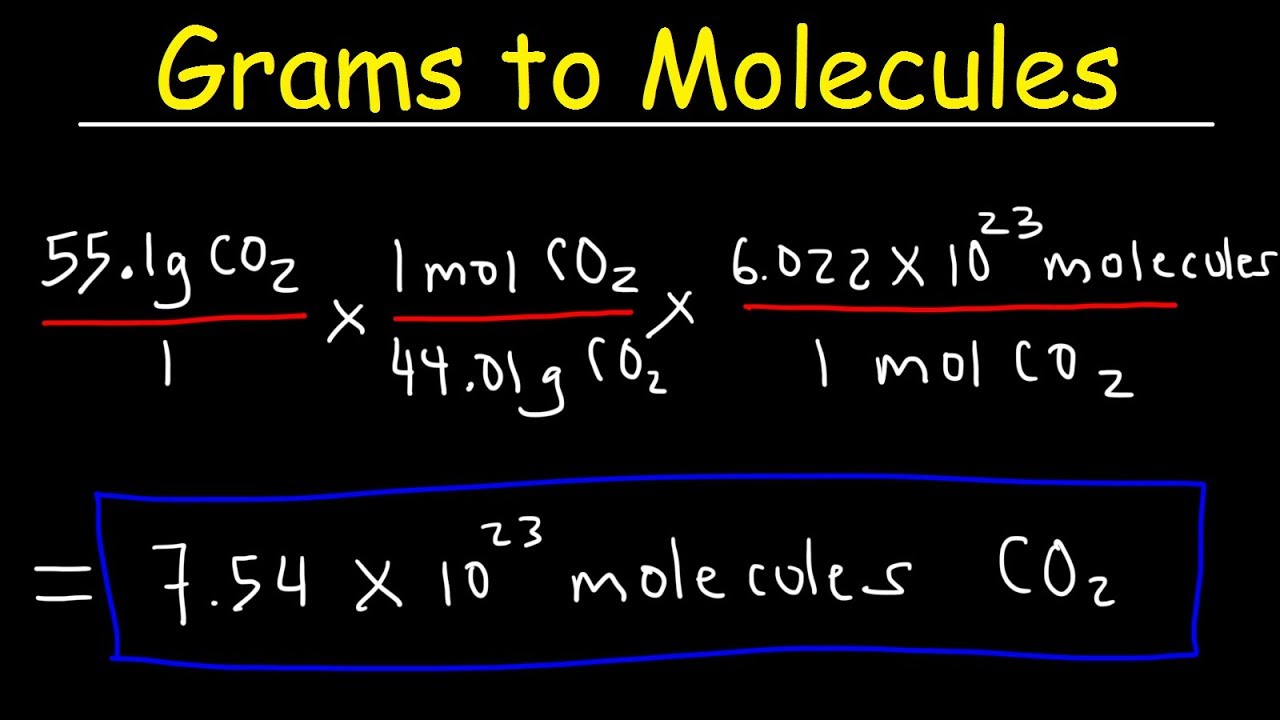
Grams And Particles Conversion Worksheets

Grams To Grams Calculator Stoichiometry

How many grams of one element have the same number of atoms as X grams