Ch3 What Shaoe Is The Structure And Hybridization Of
The carbanion has three bonding pairs and one. Keeping hybridisation of carbon atom in mind, identify the molecule which is linear: Hence, the correct answer is.
Ch3 Molecular Geometry
The lone pair of electrons is located in one of the hybrid orbitals of the (methyl carbanion) tetrahedral structure (sp 3). The carbanion has three paired bonds and one lone pair. It is trigonal pyramidal and sp3 hybridized.
A methyl group, denoted by the symbol ch3, is a functional group consisting of one carbon atom bonded to three hydrogen atoms.
What is the ch3+ lewis structure? But in ch 3, , a positive charge makes the number of available electrons 3. The shape of [c h 3 ] + is trigonal planar. Solve any question of chemical bonding and molecular.
The carbon atom in the ion ch+3 is sp2 hybridized carbon and therefore, the geometry around this atom is trigonal planar. What is the shape and bond angle of ch3? In c h 3 + , central atom c is s p 2 hybridized so they have a trigonal planar shape. It forms 3 single bonds with h to form a planar structure with sp2 hybridization.
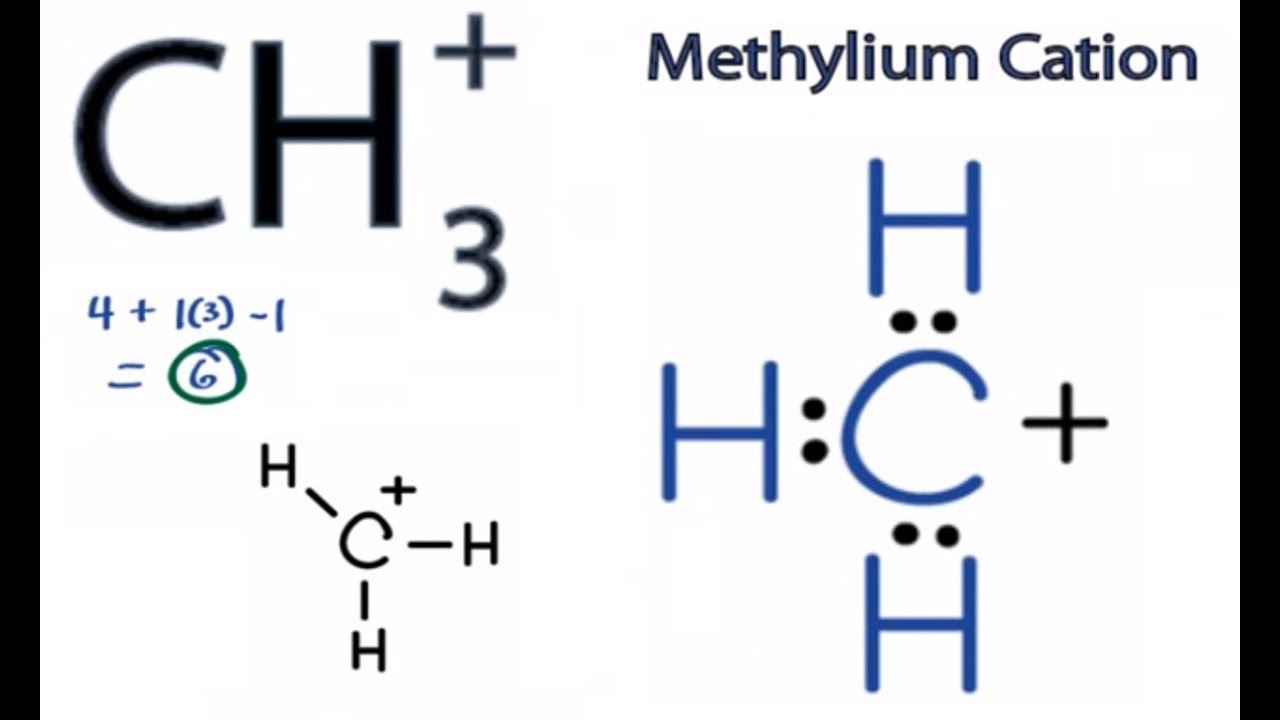
CH3+ Lewis Structure How to Draw the Lewis Structure for CH3+ YouTube
In the lewis structure of ch 3+, there are three single bonds around the carbon atom, with three hydrogen atoms attached to it, and none of the atoms has a lone pair.
What is a methyl group (ch3)?
![[CH3]](https://i2.wp.com/www.chemtube3d.com/images/gallery/inorganicsjpgs/ch3_-.jpg)
[CH3]

Ch3 Molecular Geometry

What is the structure and hybridization of CH3

SOLVED What is the the shape (molecular geometry) of CH3?