Which Is Most Likely True About Electronegativity What ?
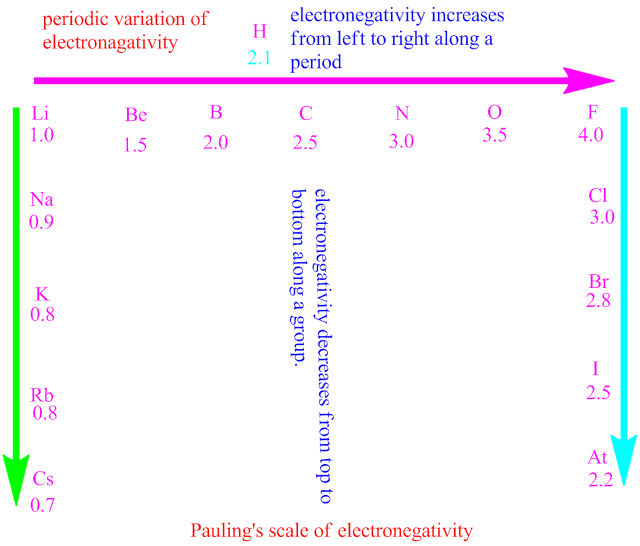
Electronegativity increases from left to right across a period due to a decrease in atomic radius and an increase in nuclear charge. Due to these trends, fluorine (f) is the most electronegative element, while cesium (cs) is generally the least electronegative. Electronegativity is important because it.
Electronegativity Difference Bond Type
How do we judge the degree of polarity? Electronegativity is a chemical property that measures how likely an atom is to attract a shared pair of electrons towards itself in a covalent bond. As we move across a period in the periodic table, the number of protons in the nucleus increases, which increases the positive charge and pulls the electrons closer to the nucleus.
Hence, fluorine is the most electronegative of the elements (not.
Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. In contrast, it decreases down a group. This knowledge helps in predicting how atoms will. In general, electronegativity increases on passing from left to right along a period and decreases on descending a group.
If we ignore the inert gases and elements for which no stable isotopes are known, we see that fluorine (\(\chi = 3.98\)) is the most electronegative element and cesium is the least. Electronegativity measures an atom's tendency to attract and form bonds with electrons. This property exists due to the electronic configuration of atoms. Electronegativity is the tendency of an atom to attract electrons when it forms a chemical bond.

What is Electronegativity Chart List of Electronegativity [PDF
It tends to increase across a period in the periodic table, meaning that.
If atoms bonded together have the same electronegativity, the shared electrons. Effective nuclear charge is directly proportional to electronegativity; During group activities, instructors expect that students will ask each other questions. Electronegativity is a measure of an atom's ability to attract the shared electrons of a covalent bond to itself.
Study with quizlet and memorize flashcards containing terms like which is most likely true about electronegativity? Electronegativity (χ) is a chemical property that describes the tendency of an atom to attract a shared pair of electrons towards itself. Electronegativity tends to be the same across a period. This statement is not true because electronegativity generally increases from left to right across a period due to an increase in the.

Electronegativity and Electronegativity Chart in PDF
Electronegativity is defined as the ability of an element or atom to attract one or.
It is true about electronegativity that it tends to increase across a period. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. Atoms with higher electronegativity attracts. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
The pauling scale is the most commonly used. The electronegativity values generally increase across a period from left to.

Electronegativity Difference Bond Type

What is Electronegativity?
Electronegativity and Periodic variation of electronegativity PG.CHEMEASY