Ch3 Geometry What Is The Structure And Hybridization Of
Ch 3+ (methenium) has one carbon atom and three hydrogen atoms. The molecular geometry of ch3 is trigonal planar. If there is one lone pair of electrons and three bond pairs the resulting.
Ch3 Molecular Geometry
Planar ch 3 (methyl free radical) possesses a sp 2 hybridization of the c atom, resulting in its planar structure. The bond angle between a carbon atom (c) and three hydrogen atoms (h) in ch3 is 109.5 degrees. Since there are no lone pairs on the central carbon atom, the molecular geometry is the same as the electronic geometry, which is trigonal planar.
So, the electronic geometry of ch3+ is.
It is trigonal pyramidal and sp3 hybridized. The resulting molecular geometry is trigonal pyramidal if there is only one pair of. Band di sini ada soal tentang bentuk geometri dari methyl ch3 tadi ada peran dengan soal yang pertanyaannya tentang masuk ke teori csr di mana di dalam teori dari. Ncert solutions for class 12 physics;
In this arrangement, the three hydrogen atoms are positioned around the central carbon atom,. Ch3+ has a trigonal planar geometry, where the three hydrogen atoms are symmetrically arranged around the carbon atom. What is the geometry of ch3+? Ncert solutions for class 12.
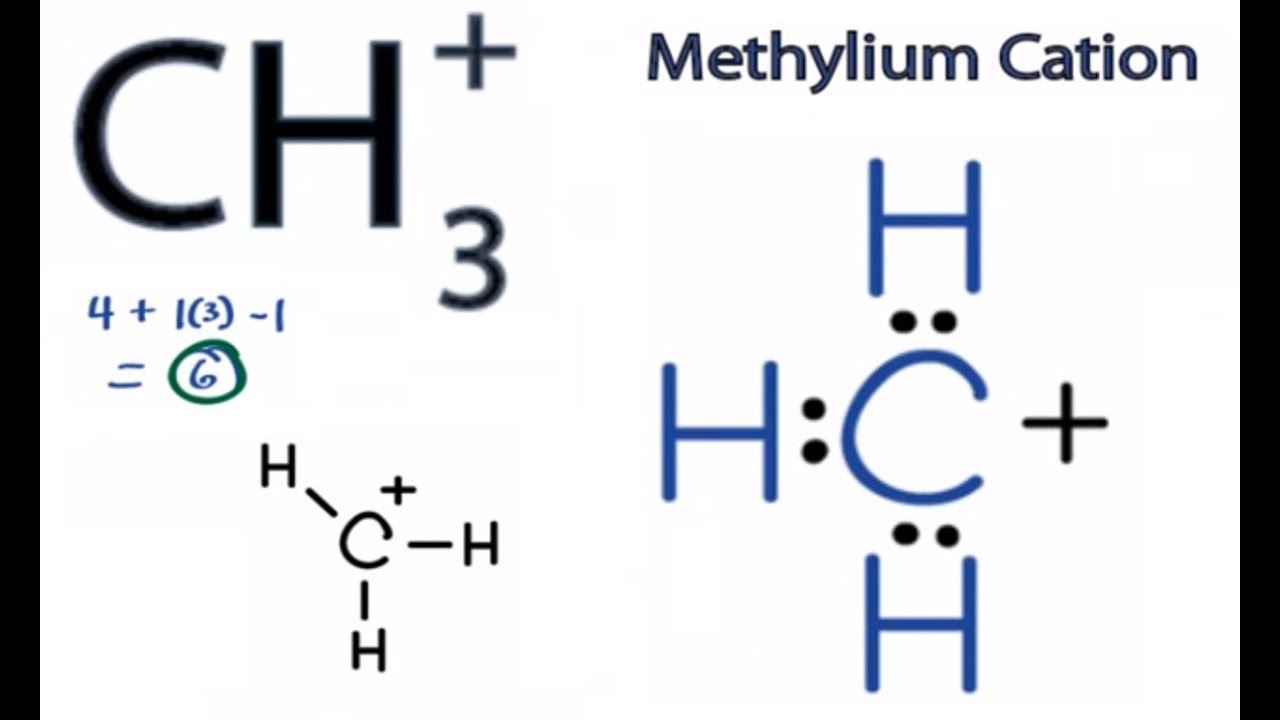
CH3+ Lewis Structure How to Draw the Lewis Structure for CH3+ YouTube
This is due to the tetrahedral geometry of the.
The lewis structure suggests that ch3⁻ adopts a trigonal pyramidal geometry. We can now use the vsepr theory to predict the molecular geometry of compounds. In addition, (methyl carbonium ion) is also planar (sp 2). If there is one lone pair of electrons and three bond pairs the.
What is the structure and hybridization of ch3 ? Draw the lewis structure of. In the lewis structure of ch 3+, there are three single bonds around the carbon atom, with three hydrogen. The electron geometry for the met.

Library of Structures Chemistry, Structures
This is because the central carbon atom is bonded to three hydrogen atoms, resulting in a flat triangular shape with bond.
We proceed through the following steps: The carbanion has three bonding pairs and one.

Ch3 Molecular Geometry

What is the structure and hybridization of CH3

According to Vsepr Theory the Molecular Geometry for Ch3+ Is En