Which Element Has 32 Protons In Its Nucleus Nucleons Atomic Number And Mass Number Defitions And More
The elements in the periodic table are arranged in the order of increasing atomic number. It has an atomic weight of 72.630 and a mass number of 74. Click here 👆 to get an answer to your question ️ which element has 32 protons in its nucleus?
Nucleons, Atomic Number and Mass Number Definitions.. and more
In this task, from the given elements we need to choose the one that has 32 32 32 protons in its nucleus. This is determined by looking at the atomic number of the elements provided. In contrast, phosphorus, cobalt, and sulfur.
The element with 32 protons is germanium.
Which element has 32 protons in its nucleus? We know the number of particles that have an element. The element with 32 protons is germanium, which has an atomic number of 32. The mass number of an atom is the.
The element with 32 protons in its nucleus is germanium (ge). The element that has 32 protons in its nucleus is germanium. The element with 32 protons in its nucleus is germanium. Since each element is identified by the number of protons in its nucleus, each element has its own atomic number, which determines the number of atomic electrons and consequently the.

SOLVED Which element has 32 protons in its nucleus? phosphorus cobalt
We know how many particles there are, we just need to identify the element.
Germanium has 32 protons in its nucleus. It explains the significance of protons in determining an element's identity and highlights. It has the chemical symbol ge and is a hard but brittle element in the carbon group. The element with 32 protons in its nucleus is germanium, which has an atomic number of 32.
Each element has a unique number of protons. We note that the number of protons in an atom's nucleus corresponds to the atomic number of the element. An element's atomic number is equal to the number of protons in the nuclei of any of its atoms. An atomic number of 32.

Which element has 32 protons in its nucleus?\ Phosphorus\ Co Quizlet
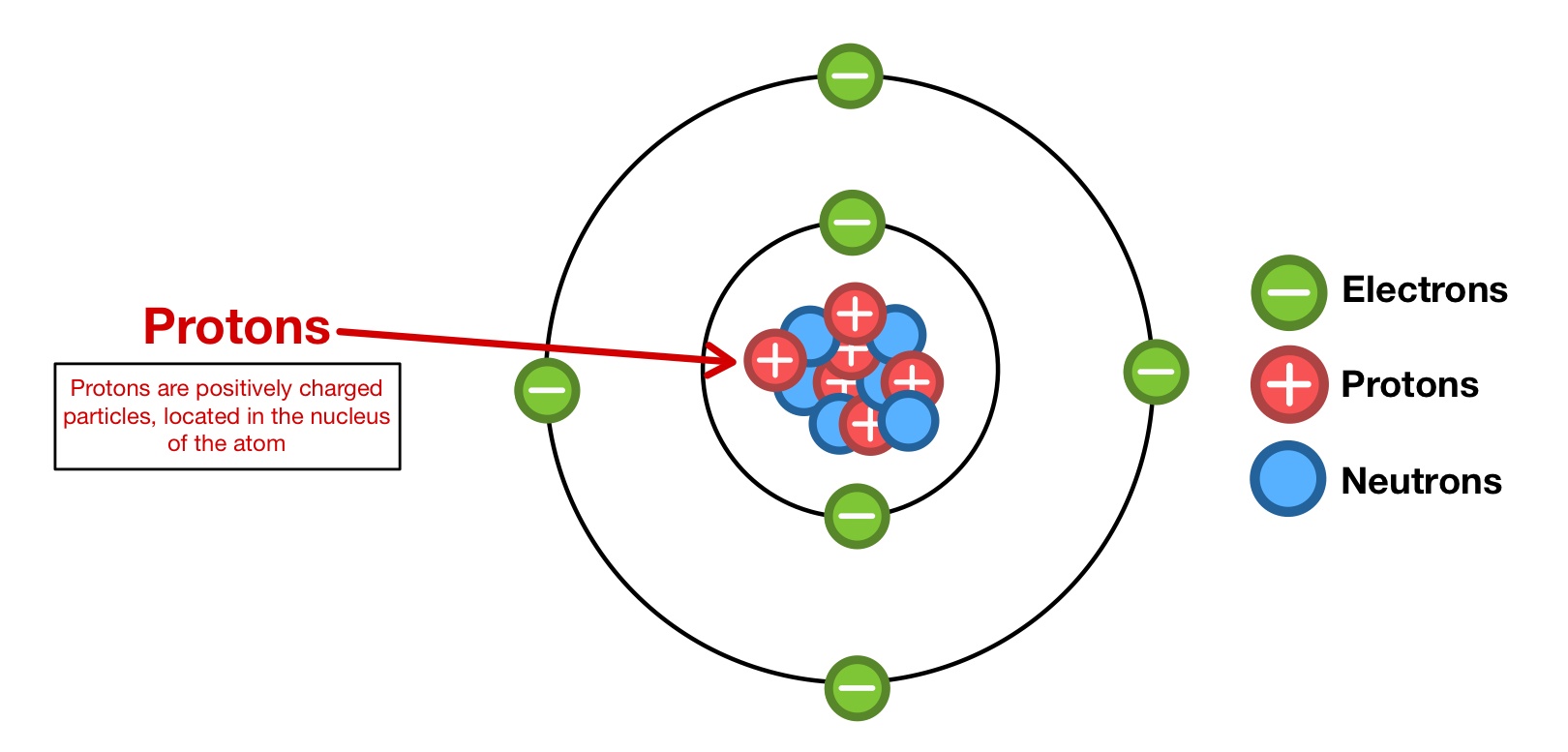
The protons, neutrons, and electrons are part of the nucleus and can't be separated.
Germanium corresponds to atomic number 32 in. Among the choices, the element with an atomic number of 32 is germanium. It is unique in having this specific number of protons, distinguishing it from other elements such as phosphorus and sulfur. In its purest form, it is also a semiconductor.
It is easy to identify the number of protons in an atom's nucleus by consulting the atomic. The protons, neutrons, and electrons are part of the nucleus, so they're out on their own. Germanium is the 32nd element in the periodic table and has a symbol of ge and atomic number of 32. According to the the nuclear model of the atom, atoms have a nucleus which is a very.

Periodic Table With Nucleon Number
Become a member and unlock all.
Other options provided have different atomic numbers. To determine which element has 32 protons in its nucleus, we refer to the periodic table where the atomic number indicates the number of protons. These two are the nucleus. This article identifies germanium as the element with 32 protons in its nucleus.
In the below table we list the number of protons, neutrons and electrons for each element in the periodic table. The atomic number of an element is the number of protons in its nucleus.

Nucleons, Atomic Number and Mass Number Definitions.. and more
Protons — Structure & Properties Expii