Caco A Covalent Of Ionic Bond Chemicl Ing Nd Covlent Compounds Britnnic
The compound itself is ionic, but in reality, it. Calcium carbonate (caco 3) is a compound with both ionic and covalent bonds. There are multiple types of chemical bonding, both inter molecular and intra molecular, that can exists between atoms.
Covalent Bonds .VS. Ionic Bonds Infogram
To determine which bond is stronger, start by identifying and listing the electronegativities of calcium (ca), carbon (c), and oxygen (o). The ionic bond between the calcium ion and the carbonate ion ensures the stability of the compound, while the polar covalent bonds within the carbonate ion contribute to. P i = 0 indicates a pure covalent bond while p i = 1 indicates a purely ionic bond.
Each f atom has three other pairs of electrons that do not participate in the bonding;
The bond between calcium (ca) and carbonate ions is ionic. Here, calcium carbonate has an ionic bond between the calcium cation (ca 2+) and the carbonate anion(co. The bond between calcium (ca) and carbonate (co₃) ions is primarily ionic. Calcium, being a metal, tends to lose two electrons to achieve a stable electron configuration.
Secondly, within the carbonate ion itself, we find polar covalent bonds. Compounds composed of ions are called ionic compounds, or salts, and their constituent ions are held together by ionic bonds: Calcium is a alkali earth metal and carbon is a non. In the compound caco₃ (calcium carbonate), the types of bonding present are:
![[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/29dc8fda-9ab0-414a-a828-0804e7fdb8e5/covalent-bonds-or-ionic-bond---teachoo.jpg)
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo
The bonding electron pair makes the covalent bond.
Firstly, there is an ionic bond present between the calcium ion (ca²⁺) and the carbonate ion (co₃²⁻). Naming covalent compounds in the previous section, the process for using a lewis structure to determine the chemical formula of a covalent molecule was presented and. Electrostatic forces of attraction between oppositely charged. They are called lone pair electrons.
To tell if caco3 (calcium carbonate) is ionic or covalent (also called molecular) we look at the periodic table that and see that ca is a metal and co3 is a. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. The ionic bond is formed between the ca2+ cation and the.
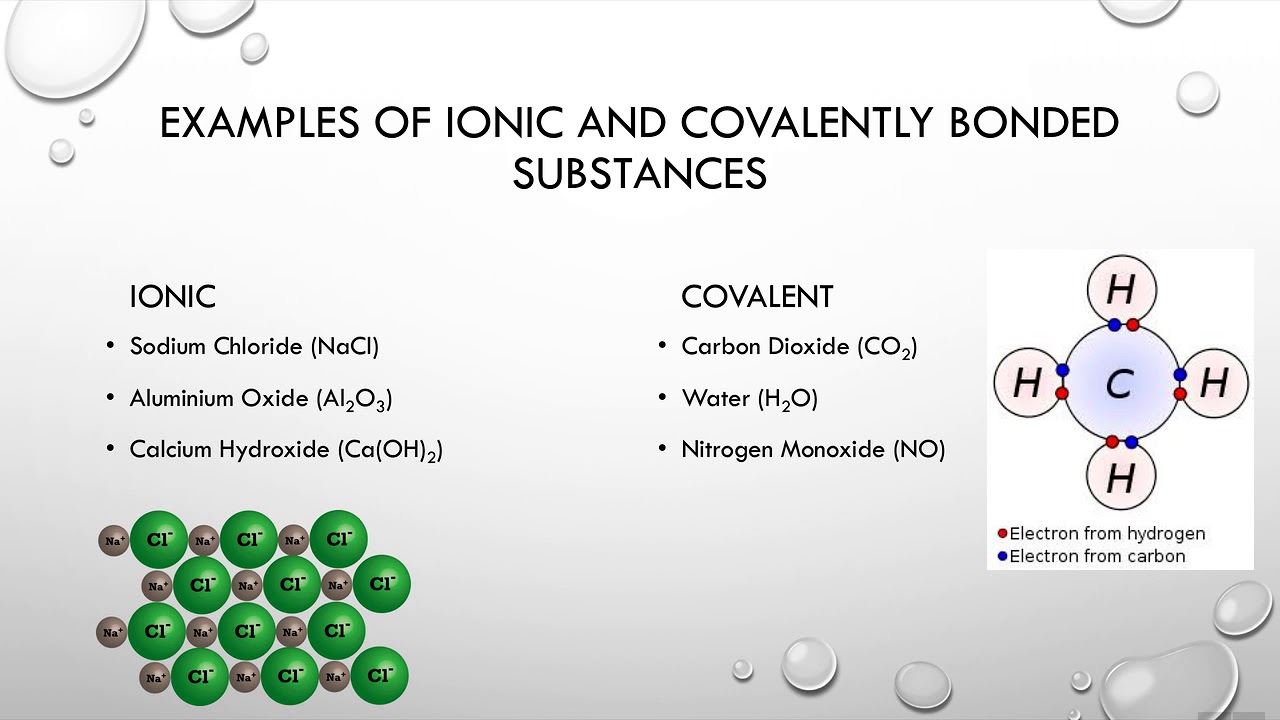
2 Ionic and covalent bonds YouTube
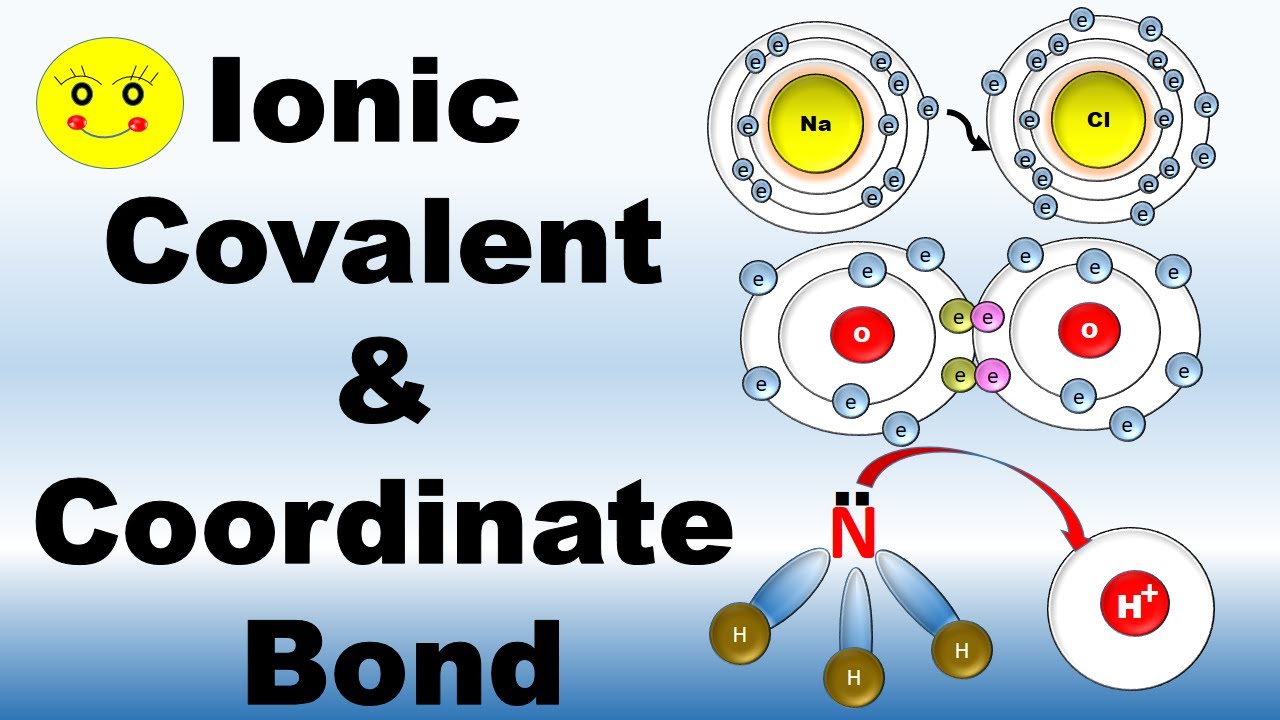
Ionic bond ,Covalent bond and Coordinate bond Chemistry Types of

chemical bonding Ionic and covalent compounds Britannica

Covalent Bonds .VS. Ionic Bonds Infogram