Chloric Acid Ionic Or Molecular Lewis Structure
A chemical formula is a concise list of the elements in a. Chloric acid contains one hydrogen atom (h), one chlorine atom (cl), and three oxygen atoms (o). Because an ionic compound is not made up of single, discrete molecules, it may not be properly symbolized using a molecular formula.
Chloric acid molecule Stock Photo Alamy
Chloric acid is molecular, as it consists of covalently bonded atoms of hydrogen, chlorine, and oxygen. Instead, ionic compounds must be symbolized by a. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures.
The formula for chloric acid is hclo3.
If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? Hydrochloric acid is a molecular compound because it is composed of covalently bonded hydrogen and chlorine atoms. When it dissolves in water, it forms ions, but in its pure. Predict which forms an anion, which forms a cation, and the charges of each ion.
Is chloric acid ionic or molecular? The combination of these elements results in the. First, is the compound ionic or molecular? Magnesium and nitrogen react to form an ionic compound.

Chloric acid molecule Stock Image F004/6242 Science Photo Library
Chloric acid (hclo3) is a strong acid as it is completely ionized in an aqueous solution, no parts of h + remain bound to it, which means the concentration of hydrogen ion.
Write the symbol for each ion and. The question is asking whether hclo3 (chloric acid) is classified as an ionic or molecular compound. Instead, ionic compounds must be symbolized by a. Instead, ionic compounds must be symbolized by a.
The inorganic compound known as chloric acid is an oxoacid compound of chlorine. Because an ionic compound is not made up of single, discrete molecules, it may not be properly symbolized using a molecular formula. Because an ionic compound is not made up of single, discrete molecules, it may not be properly symbolized using a molecular formula. You can often recognize ionic compounds because of their properties.

Chloric acid molecule Stock Photo Alamy
This will involve understanding the nature of hclo3, including its bonding and.
What is the chloric acid formula? Formulas for ionic compounds we have already encountered some chemical formulas for simple ionic compounds.

Is HClO3 (Chloric acid) Ionic or Covalent/Molecular? YouTube
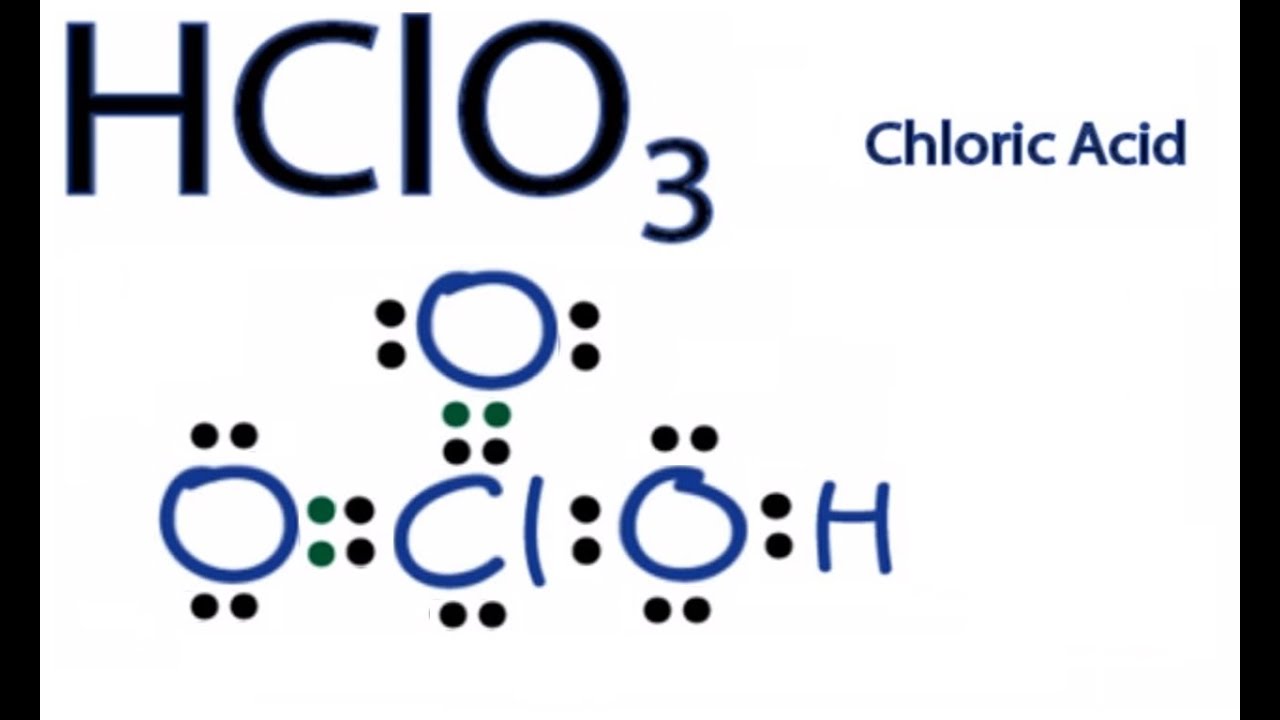
Chloric Acid Lewis Structure

Chloric Acid Molecule Photograph by Laguna Design/science Photo Library