Non Examples Of Compounds 9 4 How To Classify As Aromatic Antiaromatic And
Because it is made up of just one element , hydrogen gas (h2) is a molecule. Examples of compounds for which common names are. Br2 , bromine, is a diatomic element, or a molecular element is not an example of a compound.
Difference Between Organic and compound, Organic
It was developed by the international union of pure and. Methane (ch₄), the simplest organic compound and a natural gas. “ a compound is a substance which results from the combination of two or more chemical elements held together by a strong force between them.” let’s check some compounds that.
Atoms join together in two main ways, bonding with either an ionic bond or covalent.
The following are examples of not compounds. Br2 , bromine, is a diatomic. Only one element is in its structure though, so it is not a compound. Compounds are two different chemicals combining and forming a new substance like h2o.
Molecules are chemical combinations of two or more atoms. Example \(\pageindex{1}\) write the chemical name of sf 2, a covalent molecule that is formed when fluorine and sulfur bond with one another. The main example of compound is water represented by the chemical formula ${{h}_{2}}o$ which indicates 2 atoms of hydrogen combines with 1 atom of oxygen, sodium chloride etc and non. Compound a pure substance made from two or more elements which are chemically bonded in a fixed ratio.

Element Non Examples
Thus, the compound water has a definite composition of 11.2%.
Ch3 cooh, acetic acid, is a molecule, and is also a compound. One example is a hydrogen molecule, h2. The following are examples of not compounds. The iupac naming system is a set of rules used to give every organic compound a unique name based on its structure.
It contains three different elements. Elements are substances made up of only one type of atom, such as oxygen or gold. Mixture when two or more compounds or elements are present without being. For example, water is a compound of hydrogen and oxygen elements present in a fixed proportion 1 :

Difference Between Organic and compound, Organic
Br2 , bromine, is a diatomic element, or a molecular element.

PPT Organic Chemistry Chapter 1 Introduction to organic chemistry
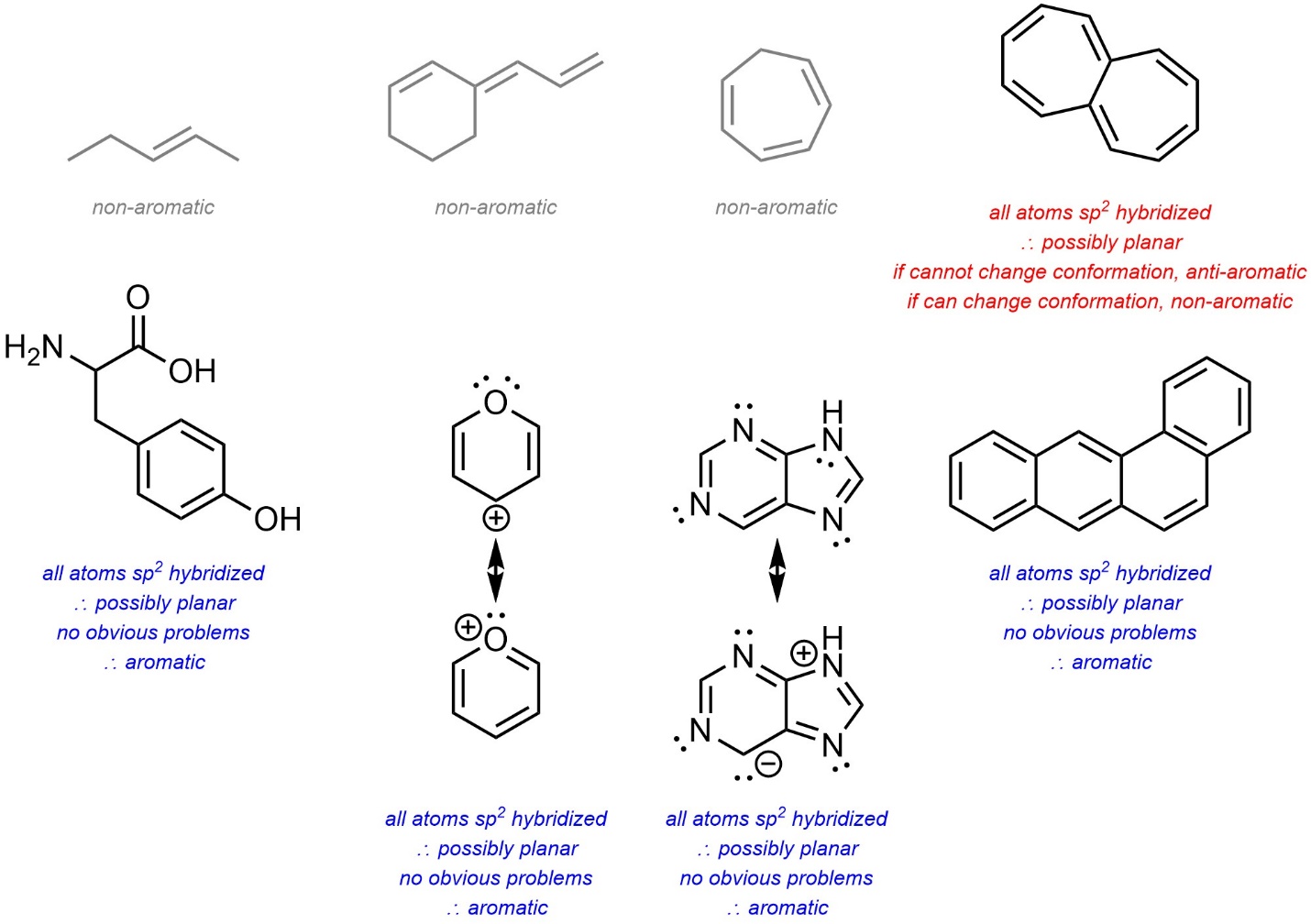
9.4. How to Classify Compounds as Aromatic, AntiAromatic, and Non

Non Aromatic Compounds Definition, Difference Between Aromatic & Non