Methylene Chloride Polar Ity
Top , references , notes data compilation copyright by the u.s. Verifying that you are not a robot. The molecule has a dipole moment, which arises from the.
What is Methylene Chloride? The Chemistry Blog
Methylene chloride is a polar molecule because it has polar bonds and a tetrahedral shape. Methylene chloride, also called dichloromethane (dcm), is a volatile, polar, organochloride compound that’s miscible with many organic solvents. Dichloromethane is an interesting compound because, despite containing two chlorine atoms, it is a polar molecule.
Methylene chloride, also called dichloromethane (dcm), is a volatile, polar, organochloride compound that’s miscible with many organic solvents.
A polar molecule with two or more polar bonds must have an asymmetric. Secretary of commerce on behalf of the u.s.a. A colorless liquid at room temperature,. As the shape of the molecule is tetrahedral and carbon and chlorine have a difference in their electronegativity.
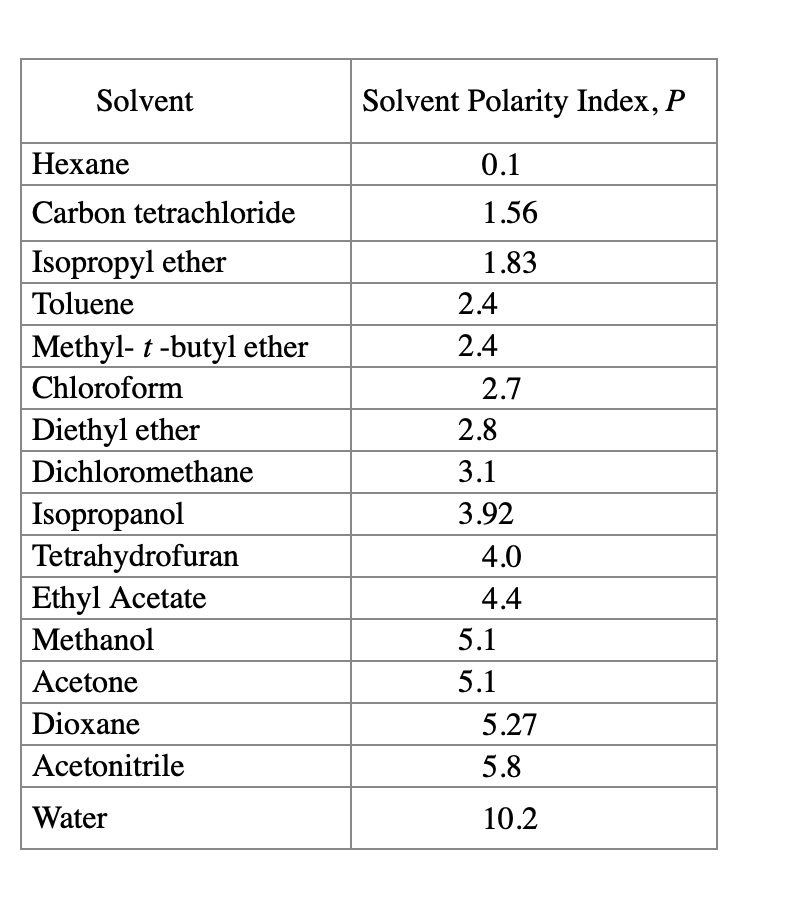
Ch2cl2 has a tetrahedral molecular structure, with a central carbon atom bonded to two chlorine atoms and two hydrogen atoms. Kovats' ri, polar column, temperature ramp go to: It is a polar, aprotic solvent, which means it has a. The electronegativity values of carbon, hydrogen, and chlorine are 2.55, 2.20, and.
Methylene Chloride Polarity
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
The ch2cl2 molecule is polar in nature. Dichloromethane (dcm), also known as methylene chloride, is a good solvent for extraction processes due to its unique properties.

SOLVED Consider methylene chloride (CHZCI2). Two equivalent Lewis

Methyl Chloride Lewis Structure

Methylene Chloride Polarity

What is Methylene Chloride? The Chemistry Blog