Which Is True About Dissolving A Polar Substance In Water Ppt Mre Biology Lesson 3 Powerpot Presenttion Free Downlod
The correct option regarding dissolving a polar substance in water is b: The solvent particles are all nonpolar molecules. Water is, therefore, what is referred to as a.
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198
Water molecules move throughout the solute. Which is true about dissolving a polar substance in water? Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can.
The dissolving process in water highlights how polar and ionic compounds like sodium chloride interact with water due to its polar nature.
The solvent particles are all nonpolar molecules. How does the polarity of water give it this solvent property? 'the solute particles are carried into the solution,' since water's polar nature allows solute particles to be surrounded. The dissolving process in water is primarily influenced by the polarity of both the solvent (water) and the solute.
The correct answer is b) because when a polar solute is dissolved in water, the solute molecules are attracted and surrounded by the polar water molecules and carried into the solution which. Polar solutes do not dissolve easily in water. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances. This statement is true because when a polar substance dissolves in water, the solute particles are surrounded by water molecules and.

Diagram The Polarity Of A Water Molecule. The Strong Polar B
A chemist dissolved crystals of an unknown substance into.
Which is true about dissolving a polar substance in water? Water molecules are attracted by solute ions at the surface of the solute. A substance capable of dissolving other polar molecules and ionic compounds. The solute particles are carried into the solution.
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. When a polar substance is dissolved in water, the solute particles get surrounded by the water molecules to form hydration shells because of strong electrostatic interactions. Water is therefore referred to as a solvent: The charges associated with these molecules form.

PPT Chapter 2 Water PowerPoint Presentation, free download ID262198
Molecules or ions of a solute spread throughout the water molecules.
When a polar substance dissolves in water, it interacts positively with the water molecules due to their polar nature. Which is true about the dissolving process in water? 1 the solute particles are carried into the solution. Water typically dissolves most ionic compounds and polar molecules.
When a polar substance dissolves in water, water molecules will surround particles of that dissolved substance. The solute particles are carried into the solution. The solute is a substance being dissolved while the solvent is what. The solute particles have no.

“Like Dissolves Like” or Water Dissolves Polar Molecules; How true is
Nonpolar molecules, such as those found in grease or oil, do not dissolve in water.

PPT Marine Biology Lesson 3 PowerPoint Presentation, free download
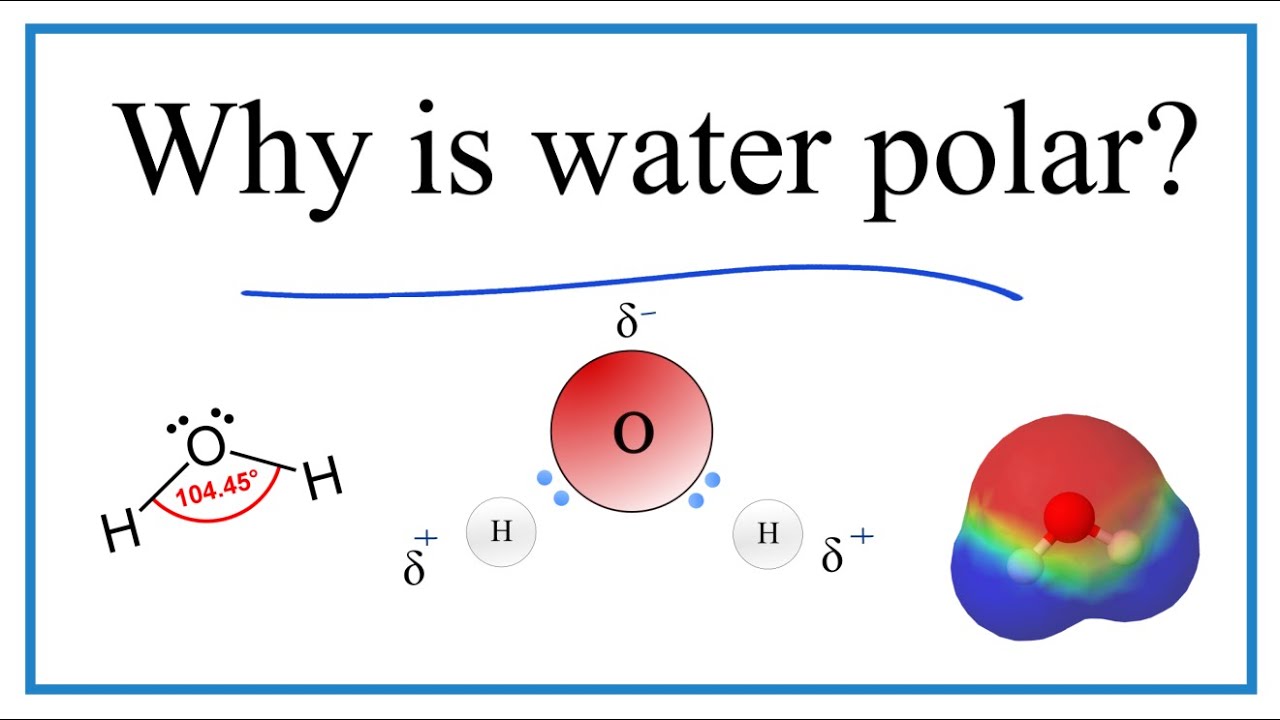
Diagram Water Interactions With Polar Molecules Describe Pol